Advertisment
European Commission approves Klisyri to treat actinic keratosis – Almirall
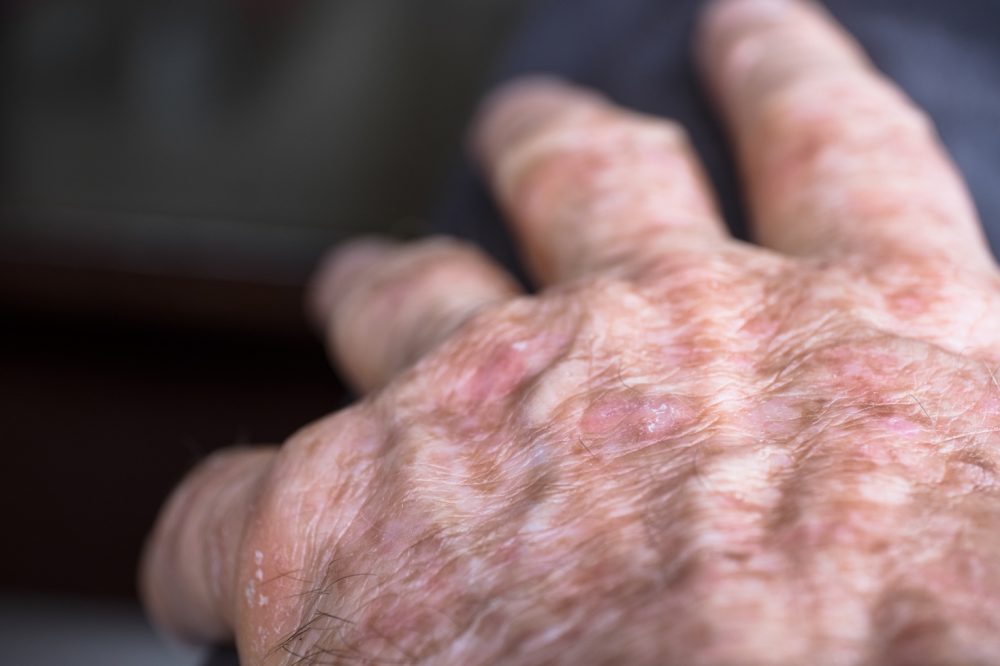
Almirall S.A. announced that the European Commission (EC) has approved Klisyri (tirbanibulin) for the topical treatment of actinic keratosis (AK) on the face or scalp in adults. Tirbanibulin is a novel, topical first-in-class microtubule inhibitor with a selective antiproliferative mechanism of action that represents a significant step forward in the treatment of AK due to its short treatment protocol -one application daily for 5 days-, proven efficacy and safety profile, with very acceptable local tolerability.
The reported prevalence of AK in the European population is around 18% and its incidence is predicted to increase globally due to an aging population, increased exposure to UV radiation, and changes in UV-seeking behaviours.
This approval is based on two pivotal phase III studies’ (KX01-AK-003 and KX01-AK-004) positive results, published in the New England Journal of Medicine (NEJM) These two double-blind, vehicle-controlled, randomised, parallel-group, multi-centre phase III clinical trials, included 702 patients from 62 clinical sites across the US, and demonstrated that once-daily application of tirbanibulin ointment 1% (10 mg/g) during 5 consecutive days in adults with AK on the face or scalp is effective and generally well tolerated. Both phase III studies achieved their primary endpoint, which was defined as 100% clearance (Complete Clearance) of the AK lesions on Day 57 in the face or scalp treatment areas, each study achieving statistical significance (p<0.0001) versus vehicle on this endpoint. In the KX01-AK-003 study, complete clearance was observed in 44% of the patients treated with tirbanibulin versus 5% for vehicle-treated groups. In the KX01-AK-004 study, complete clearance was observed in 54% of the patients treated with tirbanibulin versus 13% for vehicle-treated groups. Local reactions were mostly mild-to-moderate erythema, flaking or scaling, application-site pruritus, and application-site pain that was resolved spontaneously.
See- “Phase III Trials of Tirbanibulin Ointment for Actinic Keratosis”_ Andrew Blauvelt, M.D., Steven Kempers, M.D., Edward Lain, M.D., Todd Schlesinger, M.D., Stephen Tyring, M.D., Ph.D., Seth Forman, M.D.,
et, al.. for the Phase III Tirbanibulin for Actinic Keratosis Group”-February 11, 2021, N Engl J Med 2021; 384:512-520 DOI: 10.1056/NEJMoa2024040.





