Advertisment
CHMP recommends approval of Klisyri for actinic keratosis – Almirall
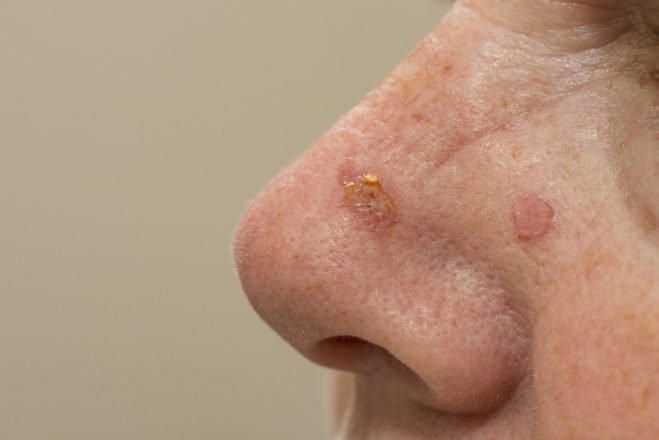
Almirall S.A. announced that the Committee for Medicinal Products for Human Use (CHMP) of the European Medicines Agency (EMA) has issued a positive opinion for the regulatory approval of Klisyri (tirbanibulin), indicated for the topical treatment of actinic keratosis (AK) on the face or scalp.
The CHMP opinion is based on two phase III studies (KX01-AK-003 and KX01-AK-004) positive results. These two double-blind, vehicle-controlled, randomized, parallel-group, multi-centre phase III clinical trials, which included 702 patients from 62 clinical sites across the US, showed that application of tirbanibulin ointment 1% (10 mg/g) in adults with AK on the face or scalp is effective and well tolerated.
Both phase III studies achieved their primary endpoint, which was defined as 100% clearance of the AK lesions at Day 57 within the face or scalp treatment areas, each study achieving statistical significance (p<0.0001) on this endpoint. In the KX01-AK-003 study, complete clearance was observed in 44% of the patients treated with tirbanibulin versus 5% for vehicle-treated groups. In the KX01-AK-004 study, complete clearance was observed in 54% of the patients treated with tirbanibulin versus 13% for vehicle-treated groups. Local reactions were mostly mild-to-moderate erythema, flaking or scaling, application-site pruritus, and application-site pain that resolved spontaneously.





